Thursday 22 December 2011
Oxidation & Reduction Process
Oxidation and reduction in terms of oxygen transfer:
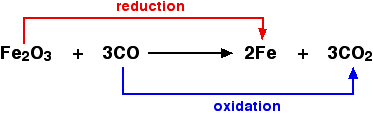 Oxidising and reducing agents An oxidising agent is substance which oxidises something else. In the above example, the iron(III) oxide is the oxidising agent. A reducing agent reduces something else. In the equation, the carbon monoxide is the reducing agent.
Definitions
For example, ethanol can be oxidised to ethanal:  | |
Ethanal can also be reduced back to ethanol again by adding hydrogen to it. A possible reducing agent is sodium tetrahydridoborate, NaBH4. Again the equation is too complicated to be worth bothering about at this point.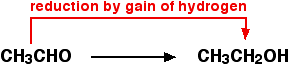
*Example of Oxidation & Reduction Process | |
Do you know anything about Voltaic Cell?? So,Let's check it out...
Voltaic Cells
In redox reactions, electrons are transferred from one species to another.If the reaction is spontaneous, energy is released, which can then be used to do useful work. To harness this energy, the reaction mus tbe split into two separate half reactions: the oxidation and reduction reactions. The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. In doing so, a Voltaic/ Galvanic Cell is created.
Introduction
When a redox reaction takes place, electrons are transferred from one species to the other. If the reaction is spontaneous, energy is released, which can be used to do work. Consider the reaction of a solid copper (Cu(s)) in a silver nitrate solution (AgNO3(s)).
2Ag+(aq) + Cu(s)  Cu2+(aq) + 2Ag(s)
Cu2+(aq) + 2Ag(s)
 Cu2+(aq) + 2Ag(s)
Cu2+(aq) + 2Ag(s)The AgNO3(s) will dissociate to produce Ag+(aq) ions and NO3-(aq) ions. The NO3-(aq) ions can be ignored since they are spectator ions and do not participate in the reaction. In this reaction, a copper electrode is placed into a solution containing silver ions. The Ag+(aq) will readily oxidize Cu(s) resulting in Cu2+(aq), while reducing itself to Ag(s).
This reaction releases energy. When the copper electrode solid is placed directly into a silver nitrate solution, however, the energy is lost as heat and cannot be used to do work. In order to harness this energy and use it do useful work, we must split the reaction into two separate half reactions; The oxidation and reduction reactions. A wire connects the two reactions and allows electrons to flow from one side to the other. In doing so, we have created a Voltaic/ Galvanic Cell.
Voltaic Cells
A Voltaic Cell (also known as a Galvanic Cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. It consists of two separate half-cells. A half-cell is composed of an electrode (a strip of metal, M) within a solution containing Mn+ ions in which M is any arbitrary metal. The two half cells are linked together by a wire running from one electrode to the other. A salt bridge also connects to the half cells. The functions of these parts are discussed below.
Figure 1 Voltaic Cell
Half Cells
Half of the redox reaction occurs at each half cell. Therefore, we can say that in each half-cell a half-reaction is taking place. When the two halves are linked together with a wire and a salt bridge, an electrochemical cell is created.
Electrodes
An electrode is strip of metal on which the reaction takes place. In a voltaic cell, the oxidation and reduction of metals occurs at the electrodes. There are two electrodes in a voltaic cell, one in each half-cell. The cathode is where reduction takes place and oxidation takes place at the anode. The figures below illustrate a cathode and an anode.
Through electrochemistry, these reactions are reacting upon metal surfaces, or electrodes. An oxidation-reduction equilibrium is established between the metal and the substances in solution. When electrodes are immersed in a solution containing ions of the same metal, it is called a half-cell. Electrolytes are ions in solution, usually fluid, that conducts electricity through ionic conduction. Two possible interactions can occur between the metal atoms on the electrode and the ion solutions.
- Metal ion Mn+ from the solution may collide with the electrode, gaining "n" electrons from it, and convert to metal atoms. This means that the ions are reduced.
- Metal atom on the surface may lose "n" electrons to the electrode and enter the solution as the ion Mn+ meaning that the metal atoms are oxidized.
When an electrode is oxidized in a solution, it is called an anode and when an electrode is reduced in solution. it is called a cathode.
Anode
The anode is where the oxidation reaction takes place. In other words, this is where the metal loses electrons. In the reaction above, the anode is the Cu(s) since it increases in oxidation state from 0 to +2.
Cathode
The cathode is where the reduction reaction takes place. This is where the metal electrode gains electrons. Referring back to the equation above, the cathode is the Ag(s) as it decreases in oxidation state from +1 to 0.
Remembering Oxidation and Reduction
When it comes to redox reactions, it is important to understand what it means for a metal to be “oxidized” or “reduced”. An easy way to do this is to remember the phrase “OIL RIG”.
OIL = Oxidization is Loss (of e-)
RIG = Reduction is Gain (of e-)
In the case of the example above Ag+(aq) gains an electron meaning it is reduced. Cu(s) loses two electrons thus it is oxidized.
Salt Bridge
The salt bridge is a vital component of any voltaic cell. It is a tube filled with an electrolyte solution such as KNO3(s) or KCl(s). The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. Without the salt bridge, positive and negative charges will build up around the electrodes causing the reaction to stop.
Flow of Electrons
Electrons always flow from the anode to the cathode or from the oxidation half cell to the reduction half cell. In terms of Eocell of the half reactions, the electrons will flow from the more negative half reaction to the more positive half reaction.
Cell Diagram
A cell diagram is a representation of an electrochemical cell. The figure below illustrates a cell diagram for the voltaic shown in Figure 1 above.
Figure 2 Cell Diagram
When drawing a cell diagram, we follow the following conventions. The anode is always placed on the left side, and the cathode is placed on the right side. The salt bridge is represented by double vertical lines (||). The difference in the phase of an element is represented by a single vertical line (|), while changes in oxidation states are represented by commas (,).
Constructing a Cell Diagram
When asked to construct a cell diagram follow these simple instructions.
Consider the following reaction:
2Ag+(aq) + Cu(s) ↔ Cu2+(aq) + 2Ag(s)
Step 1: Write the two half-reactions.
Ag+(aq) + e- ↔ Ag(s)
Cu(s) ↔ Cu2+(aq) + 2e-
Step 2: Determine the cathode and anode.
Anode: Cu(s) ↔ Cu2+(aq) + 2e-
Cathode: Ag+(aq) + e- ↔ Ag(s)
Cu(s) is losing electrons thus being oxidized. Oxidation happens at the anode. Ag+ is gaining electrons thus is being reduced. Reduction happens at the cathode.
Step 3: Construct the Diagram.
Cu(s) | Cu2+(aq) || Ag+(aq) | Ag(s)
The anode always goes on the left and cathode on the right. Separate changes in phase by | and indicate the the salt bridge with ||.
Cell Voltage/Cell Potential
The readings from the voltmeter give the reaction's cell voltage or potential difference between it's two two half-cells. Cell voltage is also known as cell potential or electromotive force (emf) and it is shown as the symbol Ecell.
Standard Cell Potential: Eocell = Eoright(cathode) - Eoleft(anode)
The Eo values are tabulated with all solutes at 1 M and all gases at 1 atm. These values are called standard reduction potentials. Each half-reaction has a different reduction potential, the difference of two reduction potentials gives the voltage of the electrochemical cell. If Eocell is positive the reaction is spontaneous and it is a voltaic cell. If the Eocell is negative, the reaction is non-spontaneous and it is referred to as an electrolytic cell.
* Examples Video of Voltaic Cell..
Tuesday 20 December 2011
Periodic Table
The periodic table of the chemical elements (also known as the periodic table or periodic table of the elements) is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus. while rectangular in general outline, gaps are included in the horizontal rows (known as periods) as needed to keep elements with similar properties together in vertical columns (known as groups), e.g. alkali metals, alkali earths, halogens, noble gases.
Saturday 29 October 2011
My Professional Development Plan
My goals:
Since, I am the student of Bachelor in Chemistry Education of UPSI. Now, I am in the semester 3. So, my major subject is Chemistry and i choose the Mathematics as my minor subject. I hope that I will improve my knowledge and skills and score both of the subjects as well as I can. I also have a strong passion to enhance my ability in the teaching field.
After my graduation soon, I will become a teacher and I hope that I will be a dedicated and competent teacher. I will improve my teaching skills in order to improve the student learning. As a teacher, we need to identify all the potency of our students because they have a different level of proficiency. I want all my student to think creatively and in a critical way in order to achieve their target and learn in the fun and comfortable learning's environment. Students is my priority and their understanding are very important to me. So, I will apply my knowledge especially in ICT field in my teaching and learning process,so that it will become more attractive and enjoyable. I also will help my student to explore and develop their various skill and share all my experiences and give a useful guidelines to them. I hope that I will be a good role model to them and their achievement is very important for me and I hope that all my students will be a successful person.
Since, I am the student of Bachelor in Chemistry Education of UPSI. Now, I am in the semester 3. So, my major subject is Chemistry and i choose the Mathematics as my minor subject. I hope that I will improve my knowledge and skills and score both of the subjects as well as I can. I also have a strong passion to enhance my ability in the teaching field.
Teaching goals:
Friday 28 October 2011
FILA Chart..
My first group's task is to make the FILA chart. It is a new experiences for me because before this, I do not know anything about the FILA chart. But,after the lecturer had explain to me more detail about that, I had learned a something new. So, today I will share some of my knowledge and experience while doing the FILA chart. Actually, what is the FILA chart???
FILA chart is actually the useful draft to solve the problems at a certain situation. For your information, F is stands for Facts, I for Ideas, L for Learning outcomes while A is stand for Actions. By using the FILA chart,we can list the facts from the problems,ideas,learning outcomes and some actions in order to solve the problems. It is very useful and make us more easier to identify and solve the problems.
Facts - sentences in the text of problems at given situations.
Ideas - issues of problems
Learning outcomes - ways to overcome the problems
Action - steps taken to solve the problems
Example of the FILA chart:
FILA chart is actually the useful draft to solve the problems at a certain situation. For your information, F is stands for Facts, I for Ideas, L for Learning outcomes while A is stand for Actions. By using the FILA chart,we can list the facts from the problems,ideas,learning outcomes and some actions in order to solve the problems. It is very useful and make us more easier to identify and solve the problems.
Facts - sentences in the text of problems at given situations.
Ideas - issues of problems
Learning outcomes - ways to overcome the problems
Action - steps taken to solve the problems
Example of the FILA chart:
Problem Scenario 1:
Rosa, a new teacher was chose to attend a workshop on Learning & Teaching organise by the district education department. In the workshop she was exposed to many things such as learning theories, teaching methods, classroom management and assessment process. At the end of the workshop each participant needs to deliver a talk according to particular topic given randomly to them. The topic given to Rosa was Smart School.
As a curriculum officer how would you help Rosa?
| Facts | Ideas | Learning Outcomes (Issues) | Actions |
| - A new teacher. - Was chosen to attend a workshop on Learning and Teaching organize by district education department. - Was exposed to many things such as learning methods, classroom management and assessment process - needs to deliver a talk at the end of the workshop. - The topic given is about Smart School. | - lacks of experiences and general knowledge. - needs to share the information with the other participants. - might be teaching in Smart School. -find information about Smart School and do preparation before the talk. - has to improved her communication skills. - enhance her self-confidence. | - What is Smart School? - What are the objectives of Smart School? - What are the concepts of Teaching and Learning in Smart School? - What are the differences between Smart School and ordinary school? - What are the roles played by the teachers, school and parents in order to achieve the objectives and goals of Smart School? - what are the challenges and benefits | - Surfing the internet to find information about Smart School. - doing researches for articles of Smart School at the library. - do the discussion with all the group members. - finish the assignment before the dateline. |
My Teaching Philosophy
I believe that most of us have our own teaching philosophy. So,today, I will share mine!
Now, I am the student of Bachelor in Chemistry Education of UPSI and my minor subject is Mathematics.When I had graduation soon, I do not necessarily teach chemistry only, but I may also be taught in mathematics or the other subjects. So, as a professional teacher, I should be always prepared and accept all the responsibilities that are given to me. I hope that I can practice all the knowledge that I've learned and shared with my students soon. I will try my best to gain a lots of experiences and knowledge especially in the field of ICT,so that my teaching and learning process will be more effective and can attract the students.
As a teacher, I will try to create a comfortable learning environment based on respect instead of fear. In order to achieve that, I must build a strong connection with my students, so that all of us can learn in a conducive and fun environment. In addition, communication is apparently an important key to an effectiveness of teaching and learning process. I have to be well-prepared for every lesson by planning my times and material efficiently to ensure that a successful lesson can be takes places. Moreover, I must also being up-to-date with the latest information ,global issues and getting hands with the latest technology which at the same times are the ways for me to improve myself.
Next, my students will becomes my main priority and I am aware that each of them has different level of proficiency. As a teacher, one of my roles is to coach and facilitates to them throughout the learning process by providing information and giving useful guidelines in order for them to achieve their goals and targets.
Lastly, I hope to impact the students that learning is a process that are never ends. For me actually, the learning process includes improving myself professionally. I also must always plan to experimental with different methods of presenting information and vary my teaching style while at the same times encouraging the critical thinking skills among the students in order to improve the learning process to become more effective and attractive.
Subscribe to:
Posts (Atom)










