Oxidation and reduction in terms of oxygen transfer:
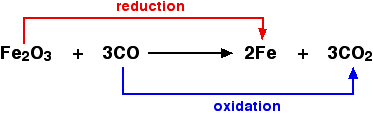 Oxidising and reducing agents An oxidising agent is substance which oxidises something else. In the above example, the iron(III) oxide is the oxidising agent. A reducing agent reduces something else. In the equation, the carbon monoxide is the reducing agent.
Definitions
For example, ethanol can be oxidised to ethanal:  | |
Ethanal can also be reduced back to ethanol again by adding hydrogen to it. A possible reducing agent is sodium tetrahydridoborate, NaBH4. Again the equation is too complicated to be worth bothering about at this point.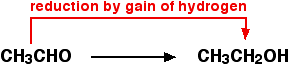
*Example of Oxidation & Reduction Process | |
Thursday, 22 December 2011
Oxidation & Reduction Process
Subscribe to:
Post Comments (Atom)



No comments:
Post a Comment